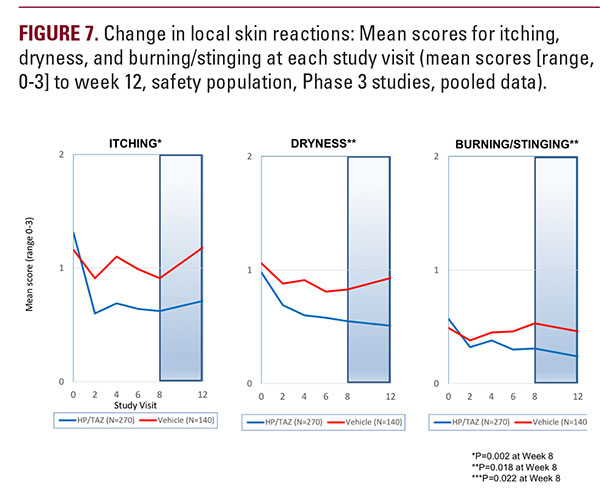
The recently published data on the phase 2 study of HP/TAZ lotion demonstrated synergistic benefits of the combination over the individual active ingredients, with a favorable irritation profile and much low incidence of application site reactions.11Here we further report the safety and tolerability of HP/TAZ lotion, presenting data on two phase 3 clinical studies in moderate-tosevere psoriasis. Treatment success was rapid and achieved in over 40% of subjects by week 8; with substantial reductions in BSA, and a significant improvement in psoriasis signs and symptoms (erythema, plaque elevation and scaling). Reduction in mean BSA was similar in those subjects with moderate and more severe disease, while there was minimal improvement with vehicle. A similar picture was seen when subjects were categorized by baseline IGA. Indeed, no subjects with severe disease who were treated with vehicle achieved treatment success.We also noted sustained improvement over the 4-week posttreatment period, likely influenced by the tazarotene component.Risk of rebound (a worsening of disease following treatment discontinuation) is an important, and often overlooked aspect of psoriasis management. Tazarotene appears to induce longer remission than TCS,9 where disease rebound after treatment discontinuation has been reported. Although the follow-up period in our studies was relatively short, a high proportion of subjects were treatment success four weeks after cessation of therapy.Treatment with HP/TAZ lotion also provided significant, rapid and sustained benefits in controlling itch, without any obvious deleterious effect of the tazarotene component which has reduced its use in psoriasis. Pruritus can be a very unpleasant and common psoriasis symptom,10 affecting sleep and impairing QoL. Itching can also occur as a result of dry skin. Skin dryness was improved by 44% and burning/stinging reduced by 46% over the 8-week treatment period and continued to improve post-treatment. Physicians continue to have long-term safety concerns with TCS,4,11,12 patients remain concerned about the risk of skin thinning, 13 and product labelling restricts halobetasol consecutive use to two weeks. There were minimal safety concerns in our two studies using an 8-week treatment regimen with HP/TAZ lotion, and long-term studies are currently being evaluated to provide useful information to patients and clinicians on the potential benefits of this topical formulation. HP/TAZ lotion was well tolerated with only three treatment related AEs reported by ≥ 2% subjects. While irritant contact dermatitis was the most common, it was not reported in the phase 2 study (with the exception of the tazarotene monotherapy arm) and here is likely the consequence of the tazarotene component in HP/TAZ lotion. In the majority of cases it was mild or moderate in severity. Pruritus was similar to the rates reported with vehicle (2.2% versus 2.9%).  Application site pain was lower than that reported in the phase 2 study (2.9% versus 3.4%), and lower than that reported with tazarotene in the phase 2 study (6.9%).8
Application site pain was lower than that reported in the phase 2 study (2.9% versus 3.4%), and lower than that reported with tazarotene in the phase 2 study (6.9%).8
 Application site pain was lower than that reported in the phase 2 study (2.9% versus 3.4%), and lower than that reported with tazarotene in the phase 2 study (6.9%).8
Application site pain was lower than that reported in the phase 2 study (2.9% versus 3.4%), and lower than that reported with tazarotene in the phase 2 study (6.9%).8CONCLUSIONS
Halobetasol propionate 0.01%/ tazarotene 0.045% lotion provides synergistic efficacy, with rapid and sustained improvement in disease severity. HP/TAZ lotion was consistently more effective than vehicle in achieving treatment success, reducing the BSA affected by the disease, and reducing erythema, plaque elevation, and scaling at the target lesion. There were minimal safety concerns with using a longer treatment than is normal for TCS options and HP/TAZ lotion may provide a realistic long-term treatment option in patients with moderate-to-severe plaque psoriasis.
DISCLOSURES
Drs Sugarman, Weiss and Stein Gold are advisors to Ortho Dermatologics; JS, LGS, JB were principal investigators in the studies; and TL, GM, RK and RI are employees of Bausch Health.
ACKNOWLEDGMENTS
We thank Brian Bulley, MSc (Konic Limited, UK) for assistance with the preparation of the manuscript. Ortho Dermatologics funded Konic’s activities pertaining to this manuscript.
REFERENCES
- Gudjonsson JE, Elder JT. Psoriasis: epidemiology. Clin Dermatol 2007;25:535-546.
- Liu Y, Krueger JG, Bowcock AM. Psoriasis: genetic associations and immune system changes. Genes Immun 2007;8:1-12.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med 2009;361:496-509.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol 2009;60(4):643-659.
- Weinstein GD, Koo JY, Krueger GG, et al. Tazarotene cream in the treatment of psoriasis: Two multicenter, double-blind, randomized, vehicle-controlled studies of the safety and efficacy of tazarotene creams 0.05% and 0.1% applied once daily for 12 weeks. J Am Acad Dermatol 2003;48(5):760-767.
- Lebwohl M, Ast E, Callen JP, et al. Once-daily tazarotene gel versus twicedaily fluocinonide cream in the treatment of plaque psoriasis. J Am Acad Dermatol 1998;38(5 pt 1):705-711.
- Kaidbey K, Kopper SC, Sefton J, et al. A pilot study to determine the effect of tazarotene gel 0.1% on steroid-induced epidermal atrophy. Int J Dermatol 2001;40:468-471.
- Schwartz E, Mezick JA, Gendimenico GJ, et. al. In vivo prevention of corticosteroid- induced skin atrophy by tretinoin in the hairless mouse is accompanied by modulation of collagen, glycosaminoglycans, and fibronectin. J Invest Dermatol 1994;102(2):241-246.
- Lebwohl MG. Breneman DL, Goffe BS, et al. Tazarotene 0.1% gel plus corticosteroid cream in the treatment of plaque psoriasis. J Am Acad Dermatol 1998;39(4 Pt 1):590-596.
- Lebwohl M, Lombardi K, Tan MH. Duration of improvement in psoriasis after treatment with tazarotene 0.1% gel plus clobetasol propionate 0.05% ointment: comparison of maintenance treatment. Int J Dermatol 2001;40(1):64-66.
- Sugarman JL, Stein Gold L, Lebwohl MG, et al. A Phase 2, Multicenter, Double-Blind, Randomized, Vehicle Controlled Clinical Study to Assess the Safety and Efficacy of a Halobetasol/Tazarotene Fixed Combination in the Treatment of Plaque Psoriasis. J Drugs Dermatol 2017;16(3):194-201.
- Koo J, Lebwohl M. Duration of remission of psoriasis therapies J Am Acad Dermatol 1999;41(1):51-59.
- Erturk IE, Arican O, Omurlu IK, et al. Effect of the pruritus on the Quality of Life: A preliminary study. Ann Dermatol 2012;24(4):406-412.
- Mueller SM, Tomaschett D, Vogt DR, et al. Topical corticosteroid concerns from the clinicians’ perspective. J Dermatol Treat 2017;28(5):464-468.
- Levin E, Gupta R, Butler D, et al. Topical steroid risk analysis: differentiating between physiologic and pathologic adrenal suppression. J Dermatolog Treat 2014;25(6):501-506.
- Charman CR, Morris AD, Williams HC. Topical corticosteroid phobiain patients with atopic dermatitis. Br J Dermatol 2000;142(5):931-936
AUTHOR CORRESPONDENCE
Jeffrey L. Sugarman MD PhD pediderm@yahoo.com






