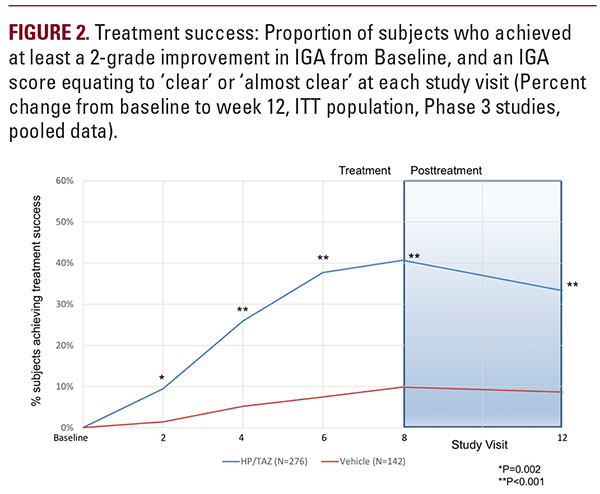
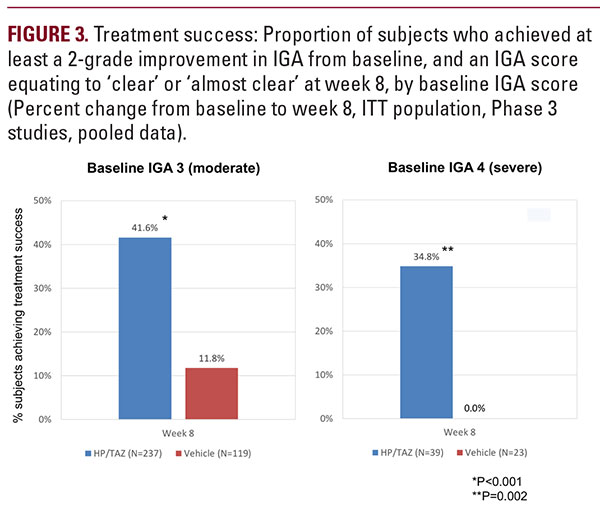
 in the IGA score, and a score of “Clear” or “Almost Clear” (treatment success). HP/TAZ lotion demonstrated statisticallysignificant superiority over vehicle as early as week 2 (P equals 0.002). By week 8, 40.7% of subjects in the HP/TAZ group achieved the primary efficacy outcome compared with 9.9% in the vehicle group (P less than 0.001; Figure 2). HP/TAZ lotion demonstrated a sustained therapeutic effect following the 4-week post-treatment period with 33.3% of subjects assessed as treatment successes at week 12, compared with 8.7% of subjects who had been treated with vehicle (P less than 0.001). Overall, 41.6% of subjects who had moderate disease (IGA = 3) at baseline were treatment successes with HP/TAZ lotion at week 8, compared with 11.8% of subjects treated with vehicle. Over a third (34.8%) of subjects with severe disease (IGA = 4) were treatment successes, with at least a 3-grade improvement in IGA. No subjects with severe psoriasis treated withvehicle achieved treatment success at week 8 (see Figure 3).
in the IGA score, and a score of “Clear” or “Almost Clear” (treatment success). HP/TAZ lotion demonstrated statisticallysignificant superiority over vehicle as early as week 2 (P equals 0.002). By week 8, 40.7% of subjects in the HP/TAZ group achieved the primary efficacy outcome compared with 9.9% in the vehicle group (P less than 0.001; Figure 2). HP/TAZ lotion demonstrated a sustained therapeutic effect following the 4-week post-treatment period with 33.3% of subjects assessed as treatment successes at week 12, compared with 8.7% of subjects who had been treated with vehicle (P less than 0.001). Overall, 41.6% of subjects who had moderate disease (IGA = 3) at baseline were treatment successes with HP/TAZ lotion at week 8, compared with 11.8% of subjects treated with vehicle. Over a third (34.8%) of subjects with severe disease (IGA = 4) were treatment successes, with at least a 3-grade improvement in IGA. No subjects with severe psoriasis treated withvehicle achieved treatment success at week 8 (see Figure 3).Severity of Psoriasis Signs (Erythema, Plaque Elevation, and Scaling) at Target Lesion Site
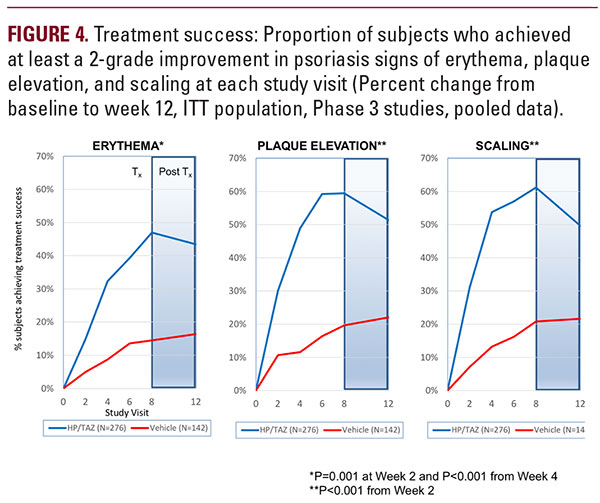
HP/TAZ lotion was statistically superior to vehicle in reducing the psoriasis signs of erythema, plaque elevation, and scaling at the target lesion. At week 8, at least a 2-grade improvement from baseline (treatment success) in severity of psoriasis signs was achieved by 47.0% (erythema), 59.5% (plaque elevation), and 61.2% (scaling) of subjects; compared with 14.5%, 19.7% and 20.8%, respectively, with vehicle (all P less than 0.001; Figure 4). HP/TAZ lotion demonstrated a sustained therapeutic effect in improving psoriasis signs four-weeks post-treatment. At week 12, treatment success was maintained in 43.4% (erythema), 51.4% (plaque elevation), and 49.6% (scaling) of subjects.BSA AssessmentHP/TAZ lotion was statistically superior to vehicle in reducing BSA. At week 8 there was a 37.6% reduction in mean BSA (P5 (mean, 8.5), there was a 39.7% reduction in BSA by week 8 that was maintained through week 12 (33.7%), compared with a 5.5% and 4.3% reduction in baseline BSA with vehicle at week 8 and 12.
Safety Evaluation
Overall, 97 subjects treated with HP/TAZ lotion reported AEs (35.9%) compared with 30 (21.4%) with vehicle (Table 3). The






