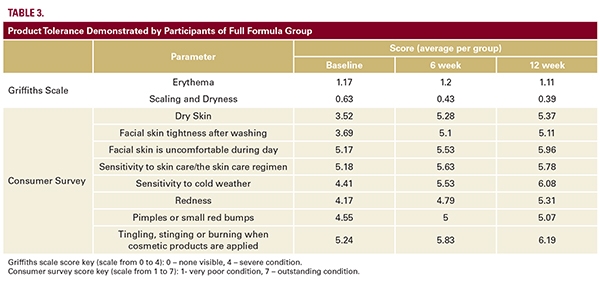
 also confirmed clinically by the excellent product tolerance; erythema, scaling, and dryness were the same in the FFG and the PG and were improved in both groups over 12 weeks (Griffiths scale) as well as via responses of the study participants on questions relating to product tolerance in consumer survey (Table 3). There was no significant change in the number of proliferating cells of either the PG or FFG, as determined by the number of positive cells in the biopsy samples stained for Ki67-protein (immunohistochemistry23; Figure 4E). In the subset of 15 patients at Site 3, dermal thickness was measured by high-resolution skin ultrasound using the Cortex system. The average dermal thickness in the FFG (11 subjects) was increased from 1.49 mm at baseline to 1.51 mm after 6 weeks and to 1.53 mm at 12 weeks (Figure 5A,Table 4). The PG (4 subjects) did not demonstrate an increased dermal thickness (average dermal thickness was 1.28 mm, 1.22 mm, and 1.26 mm at baseline, 6 weeks, and 12 weeks, respectively; Table 4). Although these numbers suggest a trend, statistical significance was not achieved. Direct measurement of hematoxylin-eosin stained samples of the skin biopsies was performed at Sites 1 and 2. One FFG participant had a >80% increase in dermal thickness (from 1.25 mm at baseline to 2.28 mm after 12 weeks of treatment; Figure 5B). No PG participants demonstrated comparable data. Clinical evaluation by Griffiths scale (42 subjects: 28 FFG and 14 PG group) revealed at least a one-grade improvement in visible pore size for the FFG vs PG at baseline compared to week 12 (P=0.036).This result was validated by the Quanti Care analysis (12 subjects: 8 FFG and 4 PG) that demonstrated a significant improvement of the skin quality associated with visible pores at baseline compared to week 12 in the FFG vs the PG (P=0.021; Figure 6A). The 3-dimensional imaging confirms this observation (Figure 6B). It was notable that the treatment with the full formula caused not only the appearance of significant reduction in the number of visible pores, but also pore size and pore depth were reduced (Figure 6B). While analyzing visible superficial wrinkles, we excluded all participants who had Griffiths Score 1 or 0 from the analysis. We surmised the moisturization provided by both the sunscreen and the cleanser may partially mask the effect from the tested treatment in mild aging. Therefore, in the analysis of superficial wrinkles using Griffiths scale we included only participants who scored 2, 3, or 4 at baseline (moderate and severe conditions). 21 participants of 25 of the FFG demonstrated at least 1 grade improvement in superficial wrinkles at week 6 or week 12 when evaluated in accordance with Griffiths scale, while in the PG only 6 participants of 12 showed any improvement. The result was statistically significant (P=0.048); the 3-dimensional- (Figure 7A, 7B) and micro- (Figure 7D) imaging confirm this observation. A clinical example of wrinkle reduction is shown in Figure 8. A combined analysis of parameters was also performed in the subgroup of patients who scored 3 or 4 at baseline (moderate- severe condition) in all: pores, superficial, and deep wrinkles. Nine participants from the FFG and three participants of the PG qualified for this inclusion criteria. While 7 of 9 participants (78%) from the FFG demonstrated improvement
also confirmed clinically by the excellent product tolerance; erythema, scaling, and dryness were the same in the FFG and the PG and were improved in both groups over 12 weeks (Griffiths scale) as well as via responses of the study participants on questions relating to product tolerance in consumer survey (Table 3). There was no significant change in the number of proliferating cells of either the PG or FFG, as determined by the number of positive cells in the biopsy samples stained for Ki67-protein (immunohistochemistry23; Figure 4E). In the subset of 15 patients at Site 3, dermal thickness was measured by high-resolution skin ultrasound using the Cortex system. The average dermal thickness in the FFG (11 subjects) was increased from 1.49 mm at baseline to 1.51 mm after 6 weeks and to 1.53 mm at 12 weeks (Figure 5A,Table 4). The PG (4 subjects) did not demonstrate an increased dermal thickness (average dermal thickness was 1.28 mm, 1.22 mm, and 1.26 mm at baseline, 6 weeks, and 12 weeks, respectively; Table 4). Although these numbers suggest a trend, statistical significance was not achieved. Direct measurement of hematoxylin-eosin stained samples of the skin biopsies was performed at Sites 1 and 2. One FFG participant had a >80% increase in dermal thickness (from 1.25 mm at baseline to 2.28 mm after 12 weeks of treatment; Figure 5B). No PG participants demonstrated comparable data. Clinical evaluation by Griffiths scale (42 subjects: 28 FFG and 14 PG group) revealed at least a one-grade improvement in visible pore size for the FFG vs PG at baseline compared to week 12 (P=0.036).This result was validated by the Quanti Care analysis (12 subjects: 8 FFG and 4 PG) that demonstrated a significant improvement of the skin quality associated with visible pores at baseline compared to week 12 in the FFG vs the PG (P=0.021; Figure 6A). The 3-dimensional imaging confirms this observation (Figure 6B). It was notable that the treatment with the full formula caused not only the appearance of significant reduction in the number of visible pores, but also pore size and pore depth were reduced (Figure 6B). While analyzing visible superficial wrinkles, we excluded all participants who had Griffiths Score 1 or 0 from the analysis. We surmised the moisturization provided by both the sunscreen and the cleanser may partially mask the effect from the tested treatment in mild aging. Therefore, in the analysis of superficial wrinkles using Griffiths scale we included only participants who scored 2, 3, or 4 at baseline (moderate and severe conditions). 21 participants of 25 of the FFG demonstrated at least 1 grade improvement in superficial wrinkles at week 6 or week 12 when evaluated in accordance with Griffiths scale, while in the PG only 6 participants of 12 showed any improvement. The result was statistically significant (P=0.048); the 3-dimensional- (Figure 7A, 7B) and micro- (Figure 7D) imaging confirm this observation. A clinical example of wrinkle reduction is shown in Figure 8. A combined analysis of parameters was also performed in the subgroup of patients who scored 3 or 4 at baseline (moderate- severe condition) in all: pores, superficial, and deep wrinkles. Nine participants from the FFG and three participants of the PG qualified for this inclusion criteria. While 7 of 9 participants (78%) from the FFG demonstrated improvement Multi-Center, Double-Blind, Vehicle-Controlled Clinical Trial of an Alpha and Beta Defensin-Containing Anti-Aging Skin Care Regimen With Clinical, Histopathologic, Immunohistochemical, Photographic, and Ultrasound Evaluation
April 2018 | Volume 17 | Issue 4 | Original Article | 426 | Copyright © April 2018
Amy Taub MD,a Vivian Bucay MD,b Gregory Keller MD,c Jay Williams PhD,c and Darius Mehregan MDd
aAdvanced Dermatology/Skinfo, Lincolnshire, IL; Northwestern University Medical School, Department of Dermatology, Chicago, IL bBucay Center for Dermatology and Aesthetics, San Antonio, TX cGregory Keller Plastic Surgery, Santa Barbara, CA dWayne State University, Monroe, MI
 also confirmed clinically by the excellent product tolerance; erythema, scaling, and dryness were the same in the FFG and the PG and were improved in both groups over 12 weeks (Griffiths scale) as well as via responses of the study participants on questions relating to product tolerance in consumer survey (Table 3). There was no significant change in the number of proliferating cells of either the PG or FFG, as determined by the number of positive cells in the biopsy samples stained for Ki67-protein (immunohistochemistry23; Figure 4E). In the subset of 15 patients at Site 3, dermal thickness was measured by high-resolution skin ultrasound using the Cortex system. The average dermal thickness in the FFG (11 subjects) was increased from 1.49 mm at baseline to 1.51 mm after 6 weeks and to 1.53 mm at 12 weeks (Figure 5A,Table 4). The PG (4 subjects) did not demonstrate an increased dermal thickness (average dermal thickness was 1.28 mm, 1.22 mm, and 1.26 mm at baseline, 6 weeks, and 12 weeks, respectively; Table 4). Although these numbers suggest a trend, statistical significance was not achieved. Direct measurement of hematoxylin-eosin stained samples of the skin biopsies was performed at Sites 1 and 2. One FFG participant had a >80% increase in dermal thickness (from 1.25 mm at baseline to 2.28 mm after 12 weeks of treatment; Figure 5B). No PG participants demonstrated comparable data. Clinical evaluation by Griffiths scale (42 subjects: 28 FFG and 14 PG group) revealed at least a one-grade improvement in visible pore size for the FFG vs PG at baseline compared to week 12 (P=0.036).This result was validated by the Quanti Care analysis (12 subjects: 8 FFG and 4 PG) that demonstrated a significant improvement of the skin quality associated with visible pores at baseline compared to week 12 in the FFG vs the PG (P=0.021; Figure 6A). The 3-dimensional imaging confirms this observation (Figure 6B). It was notable that the treatment with the full formula caused not only the appearance of significant reduction in the number of visible pores, but also pore size and pore depth were reduced (Figure 6B). While analyzing visible superficial wrinkles, we excluded all participants who had Griffiths Score 1 or 0 from the analysis. We surmised the moisturization provided by both the sunscreen and the cleanser may partially mask the effect from the tested treatment in mild aging. Therefore, in the analysis of superficial wrinkles using Griffiths scale we included only participants who scored 2, 3, or 4 at baseline (moderate and severe conditions). 21 participants of 25 of the FFG demonstrated at least 1 grade improvement in superficial wrinkles at week 6 or week 12 when evaluated in accordance with Griffiths scale, while in the PG only 6 participants of 12 showed any improvement. The result was statistically significant (P=0.048); the 3-dimensional- (Figure 7A, 7B) and micro- (Figure 7D) imaging confirm this observation. A clinical example of wrinkle reduction is shown in Figure 8. A combined analysis of parameters was also performed in the subgroup of patients who scored 3 or 4 at baseline (moderate- severe condition) in all: pores, superficial, and deep wrinkles. Nine participants from the FFG and three participants of the PG qualified for this inclusion criteria. While 7 of 9 participants (78%) from the FFG demonstrated improvement
also confirmed clinically by the excellent product tolerance; erythema, scaling, and dryness were the same in the FFG and the PG and were improved in both groups over 12 weeks (Griffiths scale) as well as via responses of the study participants on questions relating to product tolerance in consumer survey (Table 3). There was no significant change in the number of proliferating cells of either the PG or FFG, as determined by the number of positive cells in the biopsy samples stained for Ki67-protein (immunohistochemistry23; Figure 4E). In the subset of 15 patients at Site 3, dermal thickness was measured by high-resolution skin ultrasound using the Cortex system. The average dermal thickness in the FFG (11 subjects) was increased from 1.49 mm at baseline to 1.51 mm after 6 weeks and to 1.53 mm at 12 weeks (Figure 5A,Table 4). The PG (4 subjects) did not demonstrate an increased dermal thickness (average dermal thickness was 1.28 mm, 1.22 mm, and 1.26 mm at baseline, 6 weeks, and 12 weeks, respectively; Table 4). Although these numbers suggest a trend, statistical significance was not achieved. Direct measurement of hematoxylin-eosin stained samples of the skin biopsies was performed at Sites 1 and 2. One FFG participant had a >80% increase in dermal thickness (from 1.25 mm at baseline to 2.28 mm after 12 weeks of treatment; Figure 5B). No PG participants demonstrated comparable data. Clinical evaluation by Griffiths scale (42 subjects: 28 FFG and 14 PG group) revealed at least a one-grade improvement in visible pore size for the FFG vs PG at baseline compared to week 12 (P=0.036).This result was validated by the Quanti Care analysis (12 subjects: 8 FFG and 4 PG) that demonstrated a significant improvement of the skin quality associated with visible pores at baseline compared to week 12 in the FFG vs the PG (P=0.021; Figure 6A). The 3-dimensional imaging confirms this observation (Figure 6B). It was notable that the treatment with the full formula caused not only the appearance of significant reduction in the number of visible pores, but also pore size and pore depth were reduced (Figure 6B). While analyzing visible superficial wrinkles, we excluded all participants who had Griffiths Score 1 or 0 from the analysis. We surmised the moisturization provided by both the sunscreen and the cleanser may partially mask the effect from the tested treatment in mild aging. Therefore, in the analysis of superficial wrinkles using Griffiths scale we included only participants who scored 2, 3, or 4 at baseline (moderate and severe conditions). 21 participants of 25 of the FFG demonstrated at least 1 grade improvement in superficial wrinkles at week 6 or week 12 when evaluated in accordance with Griffiths scale, while in the PG only 6 participants of 12 showed any improvement. The result was statistically significant (P=0.048); the 3-dimensional- (Figure 7A, 7B) and micro- (Figure 7D) imaging confirm this observation. A clinical example of wrinkle reduction is shown in Figure 8. A combined analysis of parameters was also performed in the subgroup of patients who scored 3 or 4 at baseline (moderate- severe condition) in all: pores, superficial, and deep wrinkles. Nine participants from the FFG and three participants of the PG qualified for this inclusion criteria. While 7 of 9 participants (78%) from the FFG demonstrated improvement 





