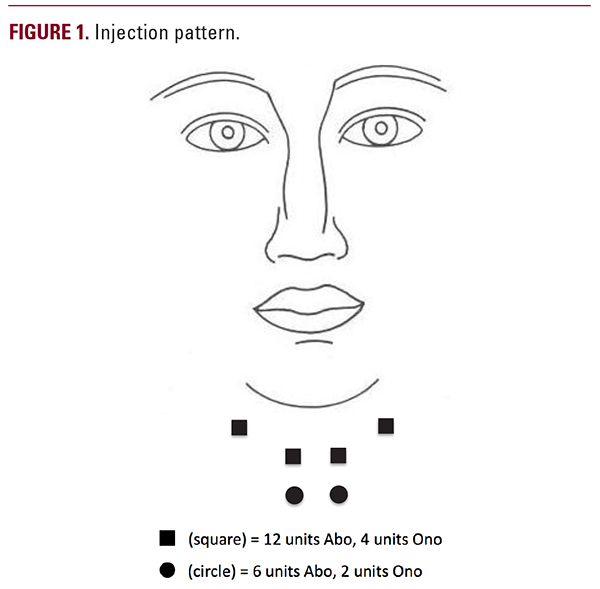
 suggested by Rystedt et al.,8 the equivalent Onabotulinumtoxin A units utilized in our patient is still only around 35 units, which is significantly below the acceptable maximum 100 Onabotulinumtoxin A units recommended in the neck and still less than the upper limit of units used by many authors.Another possibility is that our technique of injecting into the muscle belly instead of using a “bleb” technique, as done by other specialists, may cause increased diffusion to deeper structures. However, we pinch the muscle as we inject it, thus lifting it away from deeper structures making this less likely as an explanation.Lastly, inadvertent intravascular injection and subsequent spread may be a mechanism that most likely accounts for the side effects in this patient. In prior studies of Rimabotulinumtoxin B for axillary hyperhidrosis9, 7 of 20 subjects (35%) experienced mild to moderate dry mouth lasting from 2 to 38 days. Furthermore, 5 of 20 subjects (25%) experienced dry eyes lasting 2 to 128 days. The distant nature of the side effects from the treatment sites may be suggestive of systemic diffusion rather than local diffusion.Although botulinum toxin-A is generally considered a safe off-label treatment for vertical platysma bands, one should still be aware of the possible side-effects even with low dose use, as supported by our case report of dysphagia with relatively conservative doses of Abobotulinumtoxin A.
suggested by Rystedt et al.,8 the equivalent Onabotulinumtoxin A units utilized in our patient is still only around 35 units, which is significantly below the acceptable maximum 100 Onabotulinumtoxin A units recommended in the neck and still less than the upper limit of units used by many authors.Another possibility is that our technique of injecting into the muscle belly instead of using a “bleb” technique, as done by other specialists, may cause increased diffusion to deeper structures. However, we pinch the muscle as we inject it, thus lifting it away from deeper structures making this less likely as an explanation.Lastly, inadvertent intravascular injection and subsequent spread may be a mechanism that most likely accounts for the side effects in this patient. In prior studies of Rimabotulinumtoxin B for axillary hyperhidrosis9, 7 of 20 subjects (35%) experienced mild to moderate dry mouth lasting from 2 to 38 days. Furthermore, 5 of 20 subjects (25%) experienced dry eyes lasting 2 to 128 days. The distant nature of the side effects from the treatment sites may be suggestive of systemic diffusion rather than local diffusion.Although botulinum toxin-A is generally considered a safe off-label treatment for vertical platysma bands, one should still be aware of the possible side-effects even with low dose use, as supported by our case report of dysphagia with relatively conservative doses of Abobotulinumtoxin A.DISCLOSURES
REFERENCES
- Matarasso A, Matarasso SL. Botulinum A exotoxin for the management of platysma bands. Plast Reconstr Surg. 2003; 112:138S-140S.
- Carruthers A, Carruthers J. Clinical indications and injection technique for the cosmetic use of botulinum A exotoxin. Dermatol Surg. 1998; 24:1189-1194.
- Carruthers J, Carruthers A. Practical cosmetic botox techniques. J Cutan Med Surg. 1999; 3 Suppl 4:S49-52.
- Raspaldo H, Niforos FR, Gassia V, et al. Lower-face and neck antiaging treatment and prevention using onabotulinumtoxin A: The 2010 multidisciplinary french consensus--part 2. J Cosmet Dermatol. 2011; 10:131-149.
- Chen DL, Cohen JL. Botulinum toxin-A chemical denervation for platysma bands: Maximal dosing considerations. J Drugs Dermatol. 2015; 14:931.
- Carruthers J, Carruthers A. Aesthetic botulinum A toxin in the mid and lower face and neck. Dermatol Surg. 2003; 29:468-476.
- Hexsel D, Brum C, do Prado DZ, et al. Field effect of two commercial preparations of botulinum toxin type A: A prospective, double-blind, randomized clinical trial. J Am Acad Dermatol. 2012; 67:226-232.
- Rystedt A, Zetterberg L, Burman J, Nyholm D, Johansson A. A comparison of botox 100 U/mL and dysport 100 U/mL using dose conversion ratio 1: 3 and 1: 1.7 in the treatment of cervical dystonia: A double-blind, randomized, crossover trial. Clin Neuropharmacol. 2015; 38:170-176.
- Baumann L, Slezinger A, Halem M, Vujevich J, Martin LK, Black L, Bryde J. Pilot Study of the safety and efficacy of Myobloc (botulinum toxin type B) for treatment of axillary hyperhidrosis. Int J Dermatol. 2005; 44(5): 418-424
AUTHOR CORRESPONDENCE
Kseniya Golubets MD MHS kseniya.golubets@gmail.com






