

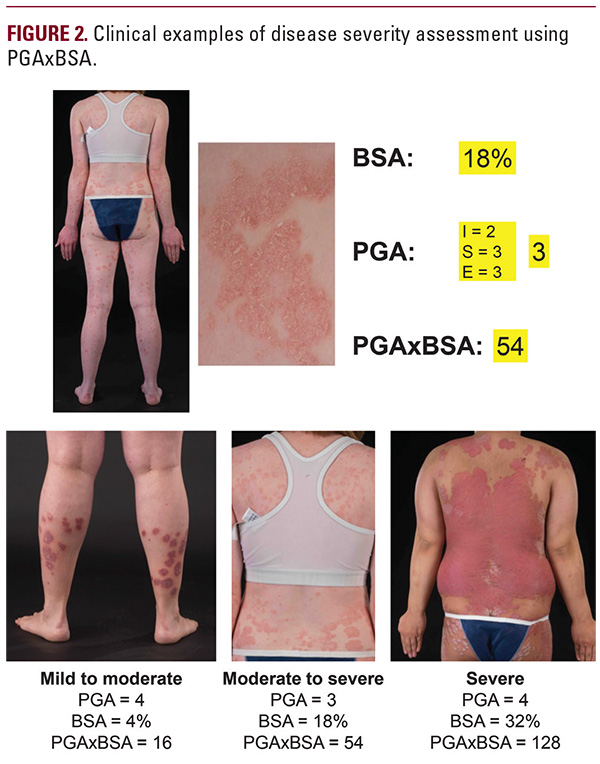
 from a clinical trial registry, the PGAxBSA assessment of disease severity correlated well with that of PASI, and demonstrated sensitivity to changes from baseline in a cohort of patients with mild to moderate psoriasis. A subgroup analysis demonstrated that PGAxBSA performed well in the subset of participants with severe disease (ie, participants with BSA ≥10%).5 These ndings suggest that PGAxBSA is a simple and effective alternative for measuring psoriasis severity compared with PASI. Apremilast (Otezla, Celgene Corporation, Summit, NJ) is an oral small-molecule phosphodiesterase 4 inhibitor that works to elevate cyclic adenosine monophosphate in immune cells, which in turn regulates the production of pro-inflammatory mediators, including tumor necrosis factor-α, interleukin-23 and interleukin-17, and anti-in ammatory mediators implicated in the pathogenesis of psoriasis.6,7 Apremilast was approved by the US Food and Drug Administration in 2014 and by the European Commission in 2015 for the treatment of adult patients with moderate to severe plaque psoriasis and adult patients with active psoriatic arthritis who are candidates for phototherapy or systemic therapy.8,9 The approval for patients with moderate to severe plaque psoriasis is based on the findings of the Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis (ESTEEM) phase 3 clinical trial program comprising 2 randomized, placebo-controlled studies that evaluated the efficacy, safety, and tolerability of apremilast 30 mg BID for the treatment of moderate to severe plaque psoriasis for up to 52 weeks, with a long-term extension to 5 years.10,11 In these studies, apremilast was well tolerated and demonstrated statistically signi cant and clinically meaningful improvements as measured by PASI-75 response at 16 weeks (primary end point), which was generally maintained in patients continuing apremilast for 52 weeks.10,11 The present post hoc analysis evaluated the PGAxBSA and PASI tools as measures of (1) psoriasis severity and (2) therapeutic response in patients receiving apremilast treatment in the phase 3 ESTEEM 1 and ESTEEM 2 clinical trials.
from a clinical trial registry, the PGAxBSA assessment of disease severity correlated well with that of PASI, and demonstrated sensitivity to changes from baseline in a cohort of patients with mild to moderate psoriasis. A subgroup analysis demonstrated that PGAxBSA performed well in the subset of participants with severe disease (ie, participants with BSA ≥10%).5 These ndings suggest that PGAxBSA is a simple and effective alternative for measuring psoriasis severity compared with PASI. Apremilast (Otezla, Celgene Corporation, Summit, NJ) is an oral small-molecule phosphodiesterase 4 inhibitor that works to elevate cyclic adenosine monophosphate in immune cells, which in turn regulates the production of pro-inflammatory mediators, including tumor necrosis factor-α, interleukin-23 and interleukin-17, and anti-in ammatory mediators implicated in the pathogenesis of psoriasis.6,7 Apremilast was approved by the US Food and Drug Administration in 2014 and by the European Commission in 2015 for the treatment of adult patients with moderate to severe plaque psoriasis and adult patients with active psoriatic arthritis who are candidates for phototherapy or systemic therapy.8,9 The approval for patients with moderate to severe plaque psoriasis is based on the findings of the Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis (ESTEEM) phase 3 clinical trial program comprising 2 randomized, placebo-controlled studies that evaluated the efficacy, safety, and tolerability of apremilast 30 mg BID for the treatment of moderate to severe plaque psoriasis for up to 52 weeks, with a long-term extension to 5 years.10,11 In these studies, apremilast was well tolerated and demonstrated statistically signi cant and clinically meaningful improvements as measured by PASI-75 response at 16 weeks (primary end point), which was generally maintained in patients continuing apremilast for 52 weeks.10,11 The present post hoc analysis evaluated the PGAxBSA and PASI tools as measures of (1) psoriasis severity and (2) therapeutic response in patients receiving apremilast treatment in the phase 3 ESTEEM 1 and ESTEEM 2 clinical trials. METHODS
Study Design and Participants
ESTEEM 1 (NCT01194219) and ESTEEM 2 (NCT01232283) were similarly designed, phase 3, multicenter, randomized, double- blind, placebo-controlled studies of apremilast 30 mg BID






