Exclusion criteria included: any past or present facial skin condition that might interfere with diagnosis or
evaluation in the study; a requirement for the use of other drugs that might enhance hyperpigmentation (hormonal and gonadotropic hormones not to be initiated during the
study); a history of increased pigmentation or contact dermatitis after using hydroquinone or tretinoin; known hypersensitivity to any ingredient in the treatment system;
participation in activities involving excessive exposure to sunlight without wearing protective clothing; and facial sunburn at the baseline visit.
The following washout periods were required: 1 week for facial hair removal; 2 weeks for facial use of
topical medications or bleaching products, photosensitizing medications or procedures, and UV light therapy or sunbathing; 3 weeks for facial use of topical tretinoin; 30 days
for participation in an investigational drug or device study; 8 weeks for facial microdermabrasion; 12 weeks for chronic use of systemic steroids; 3 months for any drug with
known potential for toxicity to a major organ; 6 months for acitretin, isotretinoin, methotrexate, photoallergic or phototoxic drugs, laser resurfacing, deep skin peels, or
injection of botulinum toxin type A or dermal fillers; and 2 years for systemic retinoids.
All patients signed informed consent, and the protocol was approved by the relevant institutional review
board.
Treatment Regimen
All patients were instructed to use the prescription strength hydroquinone/L-ascorbic acid treatment system
for normal to oily skin (Obagi-C® Rx System, OMP, Inc., Long Beach, CA) for 12 weeks. This involved using a cleansing gel, balancing toner, clarifying serum, sunscreen SPF 30,
and night cream (Table 2). The first application of the study treatment products was conducted at the end of the baseline visit under the supervision of the investigator or
their designee, and the patients were provided with verbal and written instructions on how to apply the products (Table 2).
Outcome Measures
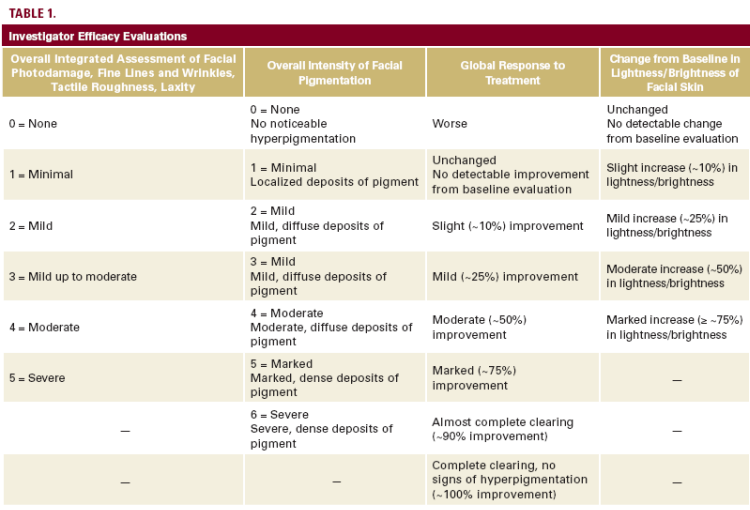
Patients were evaluated at baseline and weeks 4, 6, and 12. The investigator evaluated each patient's facial skin in terms of an overall integrated assessment of photodamage, the overall intensity of pigmentation, fine lines and wrinkles, tactile roughness,
laxity, global response to treatment, and the change from baseline in lightness/brightness (Table 1).
At each post-baseline visit, the patients were instructed to complete a questionnaire which asked them to
evaluate if the treatment system was easy to apply; their skin texture was smoother; their skin felt softer; their skin color tone was more even; their skin was more radiant;
and they had a visible reduction in fine lines and wrinkles (Ta-






