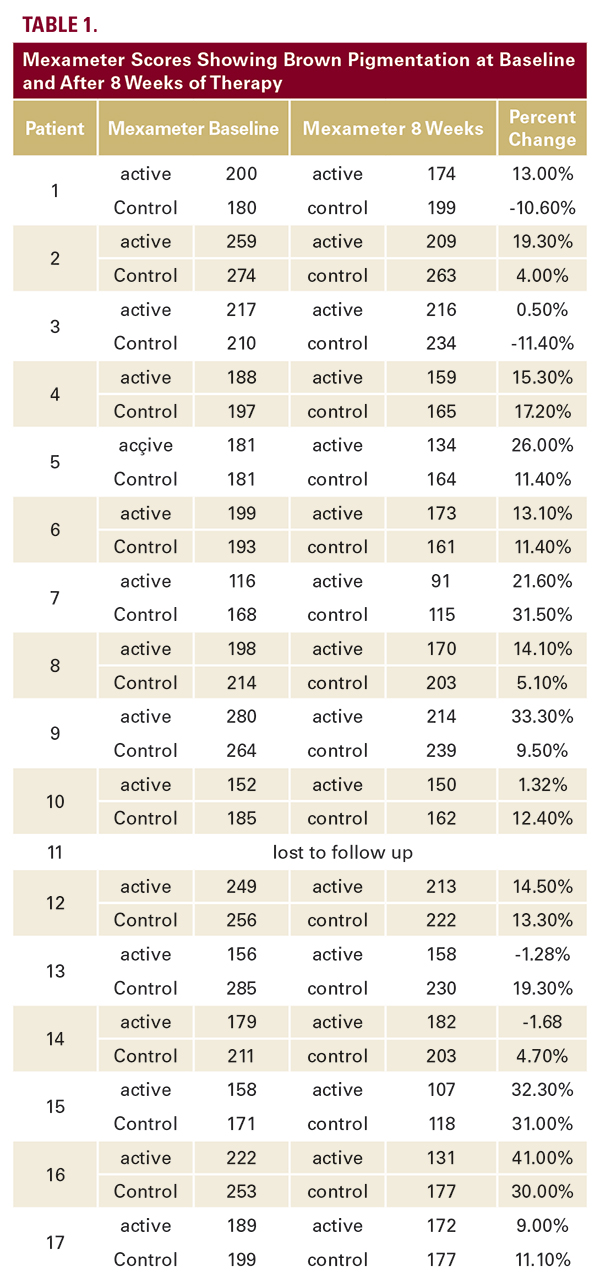
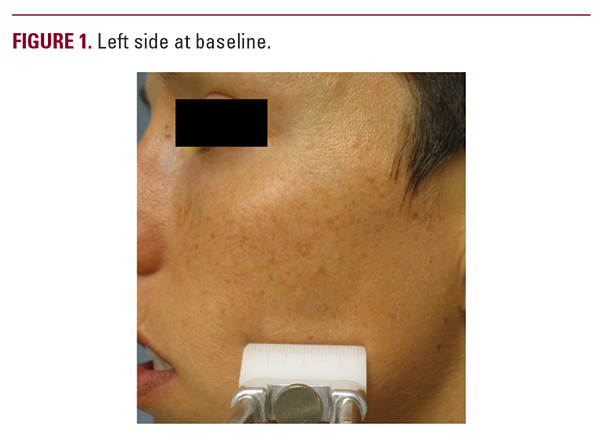
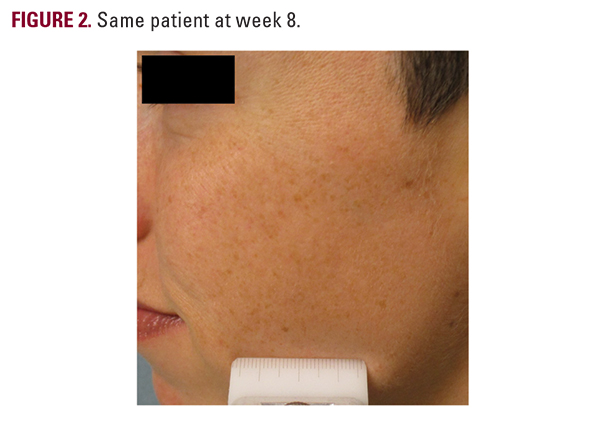
 notice one side was better than the other (Figure 1). On the physician’s assessment, 12 of 16 patients were noticed to have one side have better appearance with less hyperpigmentation. The better side was always the active cream side. On the physician’s assessment, it was also noted several patients appeared to have brighter non-hyperpigmented skin on the treated side (Figure 2).
notice one side was better than the other (Figure 1). On the physician’s assessment, 12 of 16 patients were noticed to have one side have better appearance with less hyperpigmentation. The better side was always the active cream side. On the physician’s assessment, it was also noted several patients appeared to have brighter non-hyperpigmented skin on the treated side (Figure 2).DISCUSSION
Melasma remains a frustrating condition for patients and physicians alike. LumaPro-C seems to provide significant improvement in the hyperpigmentation of melasma with no significant irritation reported. It provides an alternative to conventional therapy to those seeking a non-irritating gentle treatment. Additionally, LumaPro-C can provide an effective solution for maintaining results during necessary breaks from the use of more aggressive skin lighteners such as hydroquinone. Individuals prone to melasma are seeking solutions that will help them achieve an even skin tone without the risk of recurrence. This requires addressing existing pigmentation while also preventing future pigmentation. LumaPro-C was developed to address less severe incidences of hyperpigmentation by gently resurfacing skin. Additionally, it provides effective actives to brighten skin and reduce the manifestation of irregular discoloration caused by melanocyte hyperactivity.
DISCLOSURES
Grant support was provided by Hydropepetide®.






