According to the Statistical Analysis Plan (SAP) for each study,
the primary and secondary efficacy analyses were to be based
on an intent-to-treat (ITT) population, defined as subjects who
were exposed to study medication. Subjects who withdrew
for lack of effect were to be considered a Clinical Failure or
Treatment Failure regardless of their PGA or target lesion
scores at the last visit. Those who withdrew from either study
for reasons other than lack of effect were to be retained in the
ITT population and analyzed with the LOCF. Interim missing
data were excluded from analyses with no imputation.
The primary efficacy variables, as defined prospectively in the
protocols, were Clinical Success and Treatment Success based
on the PGA score and the TLSS, respectively.
Primary and Secondary Efficacy Results
In both Phase 3 studies, a statistically significantly greater
percentage of subjects in the desoximetasone spray 0.25%
compared to vehicle group achieved both Clinical Success and
Treatment Success at Day 28 (Figures 1 and 2). These results,
which were the primary efficacy variables, demonstrated
superior efficacy in the active study group for both overall
improvement of plaque psoriasis (by PGA) and in the individual
psoriasis lesion (by TLSS) designated at baseline as the most
severely involved plaque (target lesion).
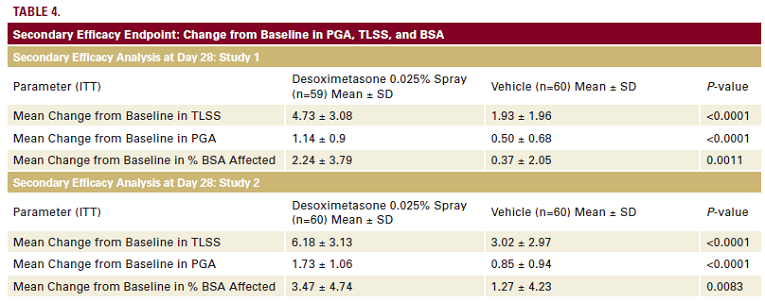
Assessment of secondary efficacy variables in both Phase 3
studies showed that subjects receiving desoximetasone Spray
0.25% twice daily exhibited statistically significantly mean changes
from Baseline to Day 28 in PGA, TLSS, and % BSA affected when
compared to subjects receiving vehicle spray twice daily (Table 4).
Safety and Tolerability Results
Tolerability and safety were assessed at all study visits. In
one study which included a total of 120 subjects, 38 subjects reported a total of 69 adverse events (AEs), with 35 AEs noted
in the active treatment group, and 34 reported by subjects in
the vehicle group.2 Among the 120 study subjects in the other
pivotal study, 21 subjects reported a total of 34 adverse events.
Of these events, 16 were reported by actively treated subjects
and 18 were noted by vehicle-treated subjects.42 Some of these
AEs were determined to be unrelated to study medication,
while others, especially application site reactions, were often
determined as possibly, probably, or definitely related to study
treatment.
Detailed summaries of AEs are from the Phase 3 studies are
depicted in Tables 5-6.4,5 No statistically significant differences
were observed between study arms and no major safety signals
related to AEs were noted. No stinging or burning was reported
in any subject throughout the study.
A total of 24 subjects discontinued from the two Phase 3 studies.
Overall, comparable proportions of subjects discontinued
from the both the active and vehicle groups within each study
and across both studies. Overall, three subjects withdrew
for insufficient therapeutic response and were considered a
Clinical Failure or Treatment Failure regardless of their PGA or
target lesion scores at the last visit. Two subjects who ran out of
study medication had BSA values of 14% and 30% at Baseline.
DISCUSSION
Desoximetasone spray 0.25% used twice daily for 28 days
proved to be effective and safe for the treatment of adults
with moderate to severe plaque psoriasis. The % BSA required
for inclusion was markedly higher in this study than in other
studies of super-potent TCS agents. In both Phase 3 studies with
desoximetasone spray 0.25%, the inclusion criteria mandated
>=10% BSA, with the mean % BSA at baseline (study entry)
ranging from 15.58%-17.78%.






