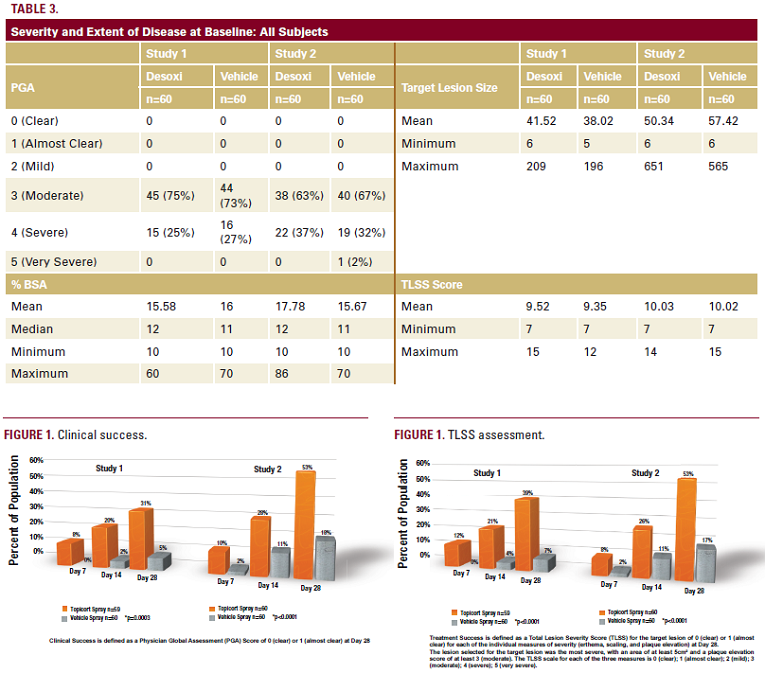
Clinical Success at Day 28 based on PGA score. The following
definitions were used to determine treatment outcome:
Clinical Success PGA score of 0 (clear) or 1 (almost clear)
Clinical Failure PGA score >1 or insufficient therapeutic
response
An insufficient therapeutic response was recorded if the
investigator noted worsening of psoriasis in a subject at any
time during the study or if a subject did not complete the study
because of lack of treatment effect, including being provided an
alternative medication or therapy for treatment of the psoriasis.
The primary endpoint based on TLSS was the proportion
of subjects in each treatment group who were considered
a Treatment Success for the target lesion at Day 28. The
following definitions were used to determine the treatment
outcome, with insufficient therapeutic response recorded as
noted above for PGA:
Treatment Success TLSS of 0 (clear) or 1 (almost clear) for
each of three individual signs: erythema,
scaling, and plaque elevation
Treatment Failure TLSS >1 for any of the individual signs or
insufficient therapeutic response






