A Phase 2 dose-finding study of desoximetasone spray in adult
subjects with moderate to severe plaque psoriasis involving
≤10% body surface area (BSA) resulted in the selection of
0.25% twice daily as the concentration and frequency of
application. A total of 151 subjects were enrolled in the fivearm
phase 2 dose-finding study, and a total of 240 subjects
collectively in the two Phase 3 trials. Table 1 depicts the three
pivotal trials, one Phase 2 trial and two Phase 3 trials, used as
the basis of submission for FDA approval.
Desoximetasone Spray 0.25% Phase 3 Pivotal Study Overview
Two Phase 3 double-blind, randomized, vehicle-controlled parallel
studies evaluated the efficacy and safety of desoximetasone
spray 0.25% twice daily versus vehicle spray twice daily for 28
days in adult patients with moderate to severe plaque psoriasis.
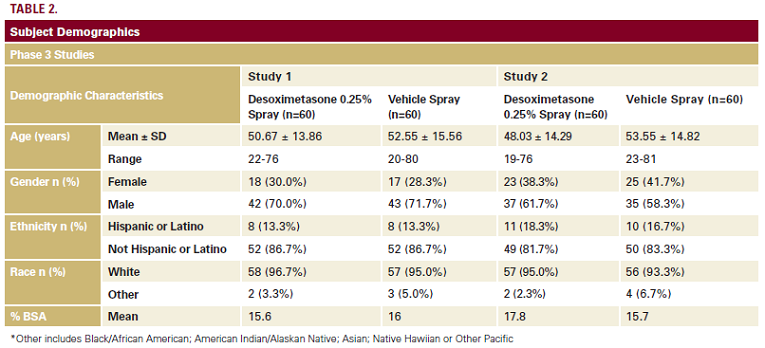
Demographic characteristics for the subjects entering both
Phase 3 studies are presented in Table 2. Appropriate candidates
for inclusion in the study had to be at least 18 years of age with
a definite clinical diagnosis of stable plaque psoriasis involving
>=10% of BSA using the “Rules of Nine†and were graded in severity
as moderate to severe by both overall assessment and with
evaluation of a designated target lesion. The mean age of the
subjects was similar across studies, ranging from 48-54 years.
There was a male prevalence and most subjects were white.
At baseline and throughout the study, the severity of disease
for the psoriatic lesions was assessed using the Physician
Global Assessment (PGA) score and a target lesion was
assessed using the Total Lesion Severity Score (TLSS), a sum of
the scores assigned by the investigator on three measures used
to determine target plaque severity, scaling, erythema, and plaque elevation (induration), with each of these criteria on a
6-point scale. A designated psoriatic plaque lesion was selected
as the target lesion upon enrollment and evaluated throughout
the study to determine the TLSS. To qualify for study entry, the
subject needed to exhibit a PGA score of 3 (moderate) or 4
(severe) for overall disease severity, and a target lesion with an
area of at least 5 cm2 that achieved a combined score TLSS of >=7,
with a plaque elevation score of >=3 (at least moderate). The mean
% BSA affected by psoriasis ranged from 15%-18% at baseline.
This and the other key disease characteristics at baseline, the
mean PGA, TLSS, and target lesion size, are presented in Table
3. There were no clinically meaningful differences in any of the
assessment values among the treatment groups
Subjects who were eligible to participate in the study based
on the inclusion and exclusion criteria and who consented
to proceed were enrolled at baseline (Day 1) and returned to
the study center at Day 7, Day 14, and Day 28 (end of study).
Education was given by study staff to subjects on proper
use of study medication with instructions to spray the study
medication directly to all affected areas and rub in gently and
completely twice-daily for 28 days. Study medication was
to be applied in the morning and evening approximately 12
hours apart. Subjects returned to the clinic for assessment of
signs and symptoms of psoriasis, adverse events, update all
concomitant medications, and to evaluate compliance with
the subject and their dosing diary.
Desoximetasone Spray 0.25% Phase 3 Study Assessments and Analyses
The primary endpoint based on PGA score was the proportion
of subjects in each treatment group who were considered a






