with psoriasis or atopic dermatitis. Topical desoximetasone
formulations, which include both mid-potency and high potency TCS
products depending on concentration and vehicle, are approved in
over fifty countries in Europe, Asia, South America, and Africa.2,3In
April 2013, desoximetasone spray 0.25% received FDA approval for
the treatment of plaque psoriasis in adults (>=18 years of age), and is
the first super-potency TCS formulation of desoximetasone.4,5
As the role of topical corticosteroid (TCS) therapy in the
treatment of several cutaneous disorders became better defined
over time, a variety of compounds and vehicle formulations
emerged.3,5,6 These CS developed for topical use are specifically
glucocorticosteroids based on the central structural nucleus of
hydrocortisone, or derivatives thereafter such dexamethasone.3,5,6
Since its introduction in 1977 of desoximetasone 0.25% cream,
subsequent formulations including 0.05% cream, gel and
ointment have been approved. While the previous 0.25%
formulations of desoximetasone are ranked as high-potency
TCS, the. 0.05% formulations are ranked as mid-potency TCS.
Desoximetasone spray was formulated using a combination
of ingredients designed to enhance cutaneous penetration
(isopropyl myristate, isopropyl alcohol), to mitigate damage to
the stratum corneum permeability barrier and provide emolliency
(glyceryloleate, mineral oil), and reduce symptoms of irritation
(l-menthol).4,5 Importantly, prior to the approval of the 0.25%
spray, desoximetasone has never been formulated to provide the
potency required to achieve the designation of super-potent TCS.
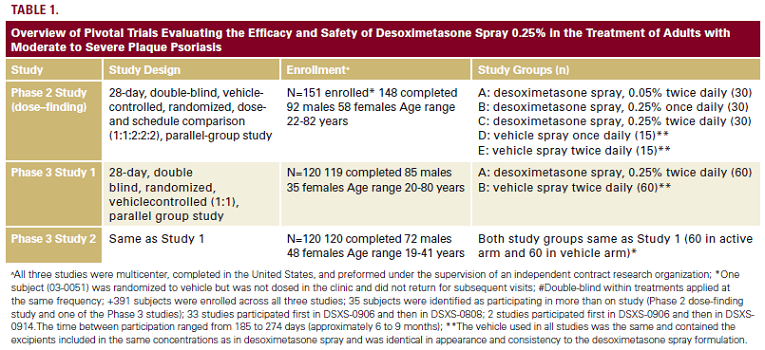
Desoximetasone Spray 0.25% Phase 1 and 2 Studies
Four pharmacologic studies were completed to support
the approval of desoximetasone spray 0.25%. A phase I vasoconstrictor assay study was completed in healthy
volunteers and demonstrated that this formulation achieved
super-high potency status.2 It was also demonstrated that
desoximetasone spray 0.25% does not induce photoallergy
or phototoxicity, is not sensitizing, and has low potential for
causing skin irritation in three additional Phase I studies. The
negligible potential for inducing skin allergenicity is supported
by the fact that desoximetasone is a Class C CS which exhibits
the lowest potential for causing cutaneous allergy among all
structural classes of TCS, calculated to be <0.2%.7,8
A phase 2 study evaluated the effects of desoximetasone spray
0.25% on hypothalamic-pituitary axis (HPA) suppression in
24 adult subjects treated twice daily for 28 days.4,5 Evaluable
cortisol levels were present in 21 subjects. Evidence of HPA
suppression was noted in 8.3% (1/12) of subjects with 10-
15% BSA and in 22.2% (2/9) of subjects with >15% BSA. In
two subjects available for follow up testing, HPA suppression
reversed within 28 days after the end of treatment.5 Plasma
levels of desoximetasone were also measured in this latter
study and were consistent with the low levels measured for the
cream and ointment formulations.4






