sessment, investigators noted better improvement in the body treatment with TriHex group.After thorough assessment of the above results, it was recognized that the subjective nature of assessments severely undermined an accurate outcome analysis. Therefore, photographic assessment was undertaken using Canfield technology, which allowed secondary objective analysis of most photographs to be completed independently by Canfield at the 8, 12, and 24-week timepoints. Thus, an objective analysis was undertaken using the following criteria:• The process involved the conversion of 2D images into a 3D space. Landmarks are placed at the top and bottom of thearm and linear distance measurements are then taken from landmark 1 to landmark 2. The same landmark placementat follow up is determined and landmarks 1 to 2 are placed and measured. The delta between baseline measurementand follow up measurement is then calculated.• All photographs assessed needed to have identical positioning, focus, and clarity so that they were comparable. This was determined by Canfield. Two patient photos in the 8-week group and 1 patient photo in the 12 and 24-week groups were deemed to be unusable.• Patients who showed worsening of outcomes with no contour improvement in both groups were labelled asnon-responders to the device. These patients could not be assessed for topical benefit as the device did not appear to cause loss of fat, which restricts the topical agent’s efficacy. This applied to 2 patients.• Thus, the final accurate assessment could be carried out by Canfield on 7 patients at 8 weeks, 8 patients at 12 weeks, and 6 patients at 24 weeks.
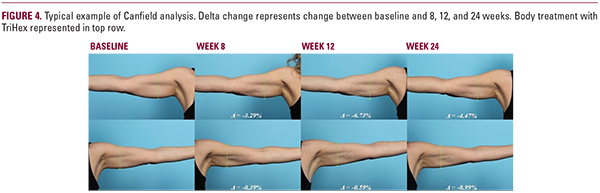
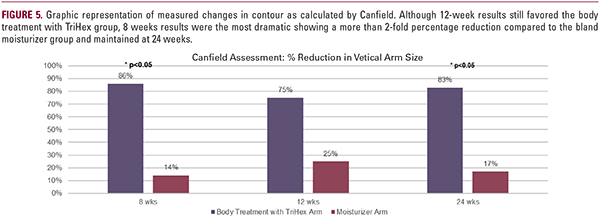
Results of Canfield Independent Assessment
Six of seven subjects (86%) assessed had improved results on the body treatment with TriHex side at 8 weeks and this persistedin 6 out of 8 subjects (75%) at 12 weeks. The delta between baseline, 8, and 12 weeks was analyzed and at the 8-week visit, body treatment with TriHex group exhibited a statistically significant difference of over twice the percent reduction from baseline compared to the bland moisturizer group. The 12- week body treatment with TriHex group also showed improved results over comparator, although not statistically significant. At 24 weeks, of the patients who returned for their visit and were suited for analysis, five of six (83%) had improved results on the body treatment with TriHex side and showed a statisticallysignificant improved reduction in contour (Figures 4, 5).