Crowdsourcing is a novel method of medical data collection,
where patients go online to submit data regarding their disease symptoms and responses to treatments.1-4 Crowdsourcing research data has potential advantages of high-volume, low-cost data collection that reflect real-world patient experiences.
However, no study to date in the dermatology literature has examined how data from crowdsourcing sites from real-world settings compare to findings from clinical trials. We examined how crowdsourced data from eczema patients compare to randomized
controlled studies.
We obtained crowdsourced data from the well-known crowdsourcing
site CureTogether (www.curetogether.com) prior to June 27, 2011. For comparison to published clinical trial data, we searched Medline for systematic reviews and meta-analyses
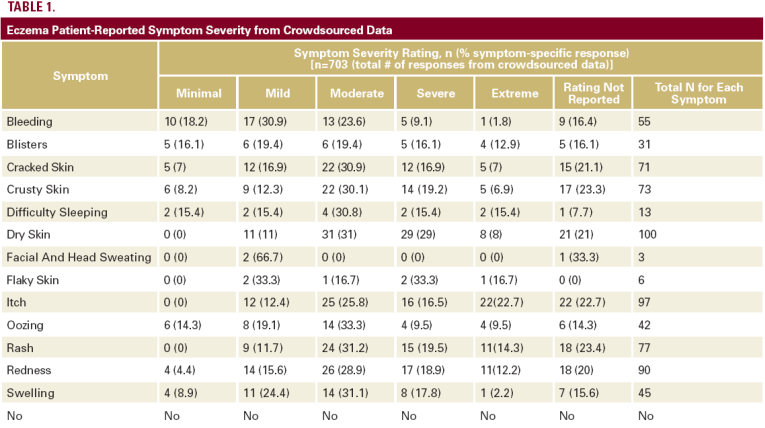
examining treatments for eczema from July 15, 2008 to July 15, 2011. We compared the clinical trial results to 246 online eczema
patients who provided 703 complete symptom responses and 538 treatment responses (Tables 1 and 2).
With regard to the use of topical corticosteroids in eczema,5 from the crowdsourcing site, 70% (54 out of 77) of the online patients reported improvement with topical corticosteroids (strength unspecified on site). In general, the crowdsourced
data were similar to the efficacy rates reported in the published literature for mid-potency topical steroids.6 Approximately 62% (48 out of 78) of the online patients reported improvement with frequent moisturization.
Comparison of published data to crowdsourced data for response
rates to topical calcineurin inhibitors was limited due to the small number of responses through the crowdsourcing site. Specifically, 67% (4 out of 6) of the online patients reported improvement
with pimecrolimus, and 100% (4 out of 4) of patients reported improvement with tacrolimus ointment. In comparison,
44% of patients achieved clearance of hand eczema after four weeks with tacrolimus 1% ointment.7 In a separate study, 28% of a pimecrolimus treatment group achieved clear or almost
clear after 22 days of treatment.7
In terms of phototherapy response rates, none of the four online
patients who underwent medical phototherapy reported symptom improvement. However, 50% (9 out of 18) of patients reported improvement when they used non-medical tanning. The published data showed phototherapy to be more effective than what was reported by a small number of online patients. For example, trial results showed that NB-UVB treatment produced
a 5-point greater reduction in disease severity compared to UVA treatment and a 9.4-point greater reduction compared to visible light treatment.8
Systemic antihistamines are used to reduce pruritus in eczema patients. Forty-seven online patients reported using systemic
antihistamines for their eczema. Two percent (1 out of 57)






